Insights,
Insights,
Since the 340B Drug Pricing Program began in 1992, covered entities’ participation has been contingent on compliance with statutory program requirements.
In fiscal year (FY) 2012, Health Resources and Services Administration (HRSA) began conducting audits of covered entities.[1] In 2012, sales at the 340B price totaled $6.9B.[2] In 2021 (the most recent data available), sales at the 340B price reached a record $43.9B.[3] This represents an average annual increase of almost 23 percent between 2012 and 2021.[4] The number of covered entity sites experienced dramatic growth, increasing from less than 20,000 covered entity sites in 2012 to more than 50,000 in 2023.[5] Despite this growth, HRSA has continued to audit around 200 covered entities per year since FY 2015.[6]
ADVI Health analyzed HRSA’s FY 2021 covered entity final audit results to assess covered entity compliance.[7] ADVI analyzed the audit findings at the national and state levels. ADVI found the following:
HRSA’s audits focus on several areas of covered entity compliance, including 340B/Medicaid duplicate discounts,[9] medicine diversion,[10] and data submission and reporting errors and inaccuracies.
It should be acknowledged that it is not clear what standards HRSA and its audit contractors are currently using to assess covered entity compliance. Following a legal challenge in 2019, HRSA “concluded that in the absence of binding and enforceable regulations, the agency would no longer issue findings based solely on noncompliance with guidance.”[11]
This determination has far-reaching implications for 340B program integrity. As a result of this policy change, HRSA stated there were 36 instances during its FY 2019 audits that did not result in a finding of noncompliance but would have resulted in one in the past.[12] The lack of clarity around program requirements and limited oversight has been well documented by the Government Accountability Organization (GAO), which has previously stated that “HRSA’s oversight of the 340B program is inadequate.”[13]
Despite these significant limitations on HRSA’s audit capabilities, ADVI’s analysis still found 62 percent of covered entities audited by HRSA in FY 2021 were non-compliant. The most common sanction imposed on these covered entities was a requirement that they repay affected manufacturers’ discounts for which the covered entities were not eligible. Covered entities were not required to pay penalties, nor did HRSA terminate any covered entities from the 340B program, as a result of noncompliance identified during these audits.
The following tables break out ADVI’s analysis of HRSA’s FY 2021 audit results on a national and state level.
Table 1: ADVI’s Analysis of National Level FY 2021 HRSA Audit Results

*Covered entities can have more than one adverse finding
Table 2: ADVI’s Analysis of State Level FY 2021 HRSA Audit Results and 2023 340B Covered Entity Registrations
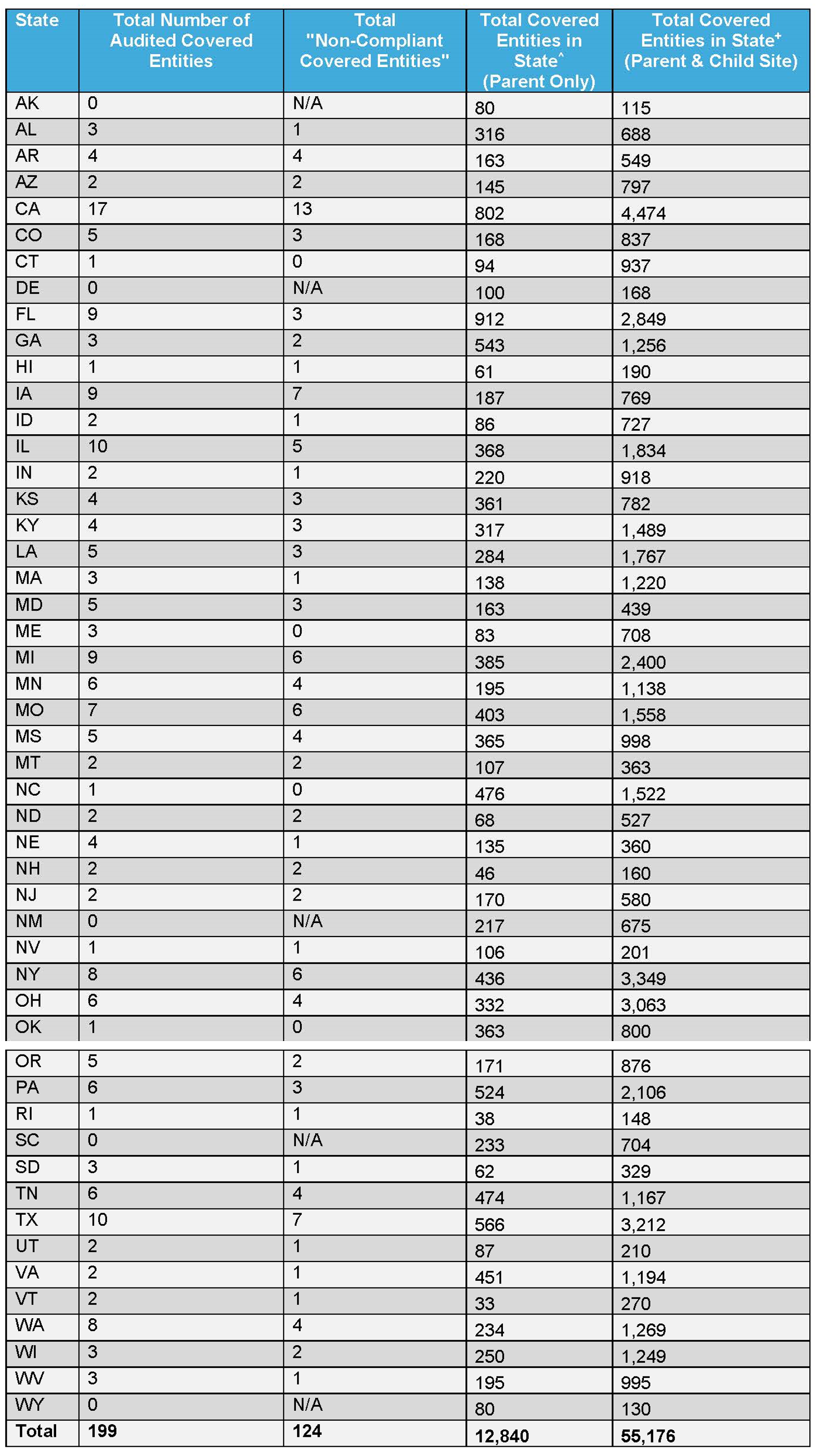
^ADVI analysis of the HRSA Office of Pharmacy Affairs (OPA) Database, 340B covered entities participating as of 1/31/2023. Analysis is at the parent level, identified based on “340B ID.” Does not take into account registrations/de-registrations throughout a year.
+ADVI analysis of the HRSA Office of Pharmacy Affairs (OPA) Database, 340B covered entities participating as of 1/31/2023. Analysis is at the individual site level (includes parent and child sites), identified based on “340B ID.” Does not take into account registrations/de-registrations throughout a year.
Funding for this research was provided by Pharmaceutical Research and Manufacturers of America. ADVI Health retained full editorial control.


Head of Policy, Research, and Analysis