Insights,
Insights,
ADVI Health analyzed the 100% Medicare FFS Inpatient Research Identifiable Files (RIFs) from November 2, 2020 through August 2022 to determine the utilization of approved NCTAP products and payment over time. Inpatient claims were selected for the analysis if they had an ICD-10 diagnosis code for COVID-19 (U07.1) anywhere on the claim. Specifically, ADVI analyzed Veklury® (remdesivir), COVID-19 convalescent plasma, and Olumiant® (baricitinib) volumes across the 22-month period and by quarter1. Additionally, ADVI analyzed changes to NCTAP product utilization over time as well as by patient severity2. Severe cases are defined as claims involving an intensive care unit (ICU) stay, mechanical ventilation/invasive mechanical ventilation/extracorporeal membrane oxygenation (IMV/ECMO), and/or supplemental oxygen (not mutually exclusive).
Key Findings
Summary
Despite oscillations in the number of inpatient COVID-19 hospitalizations, the use of COVID therapies eligible for New COVID-19 Treatments Add-on Payment (NCTAP) peaked in Q3 2021 and have been declining since (Figure 1).
Figure 1: Medicare FFS COVID-19 Inpatient Case Volume and Percent of Cases with NCTAP Product Used (Q4 2020 – Q3 2022)
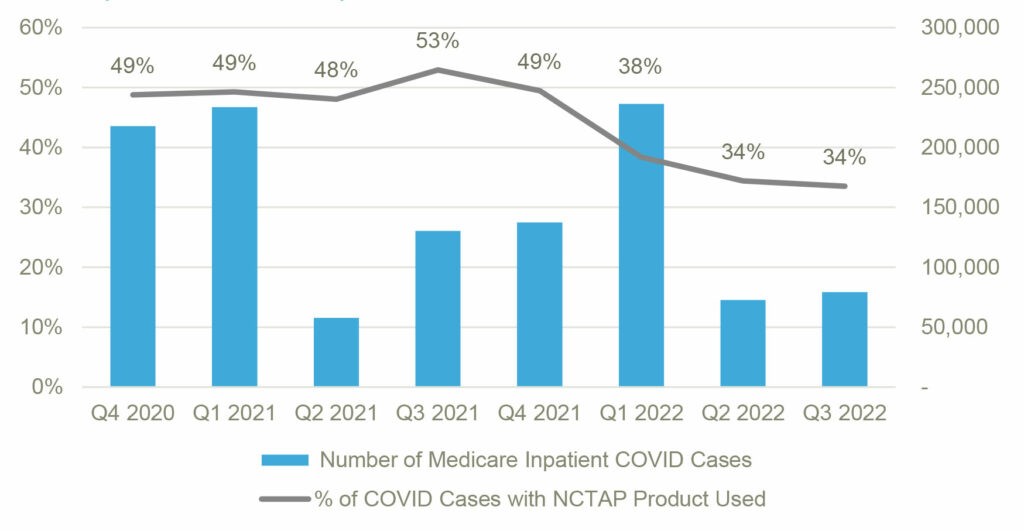
However, Figure 2, below, shows that the rate at which hospitals are receiving NCTAP payments remains static over the eight quarters analyzed, despite COVID-19 surges and increased utilization of NCTAP products.
Figure 2: Frequency of Claims with NCTAP Products vs. NCTAP Payment Rate
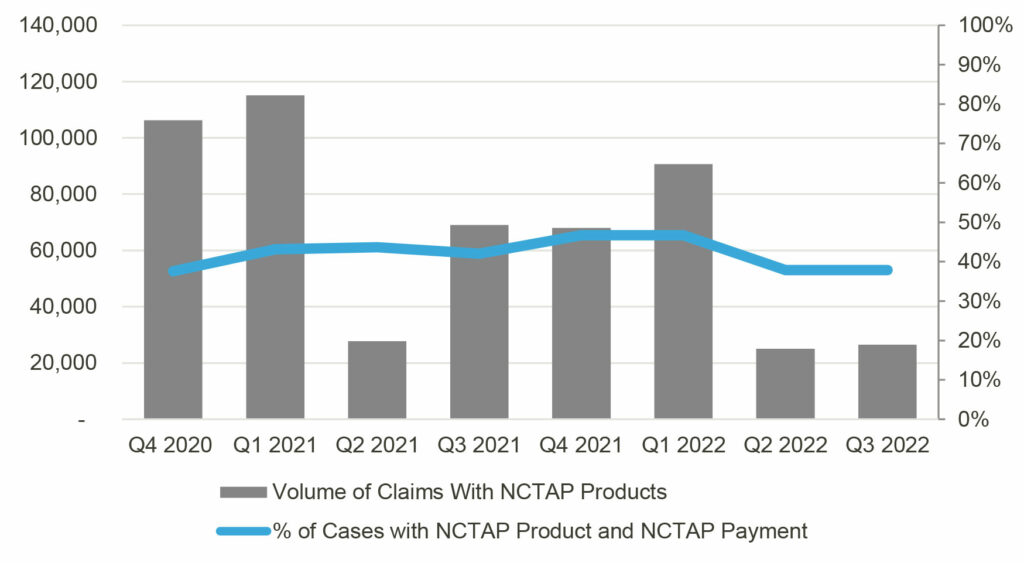
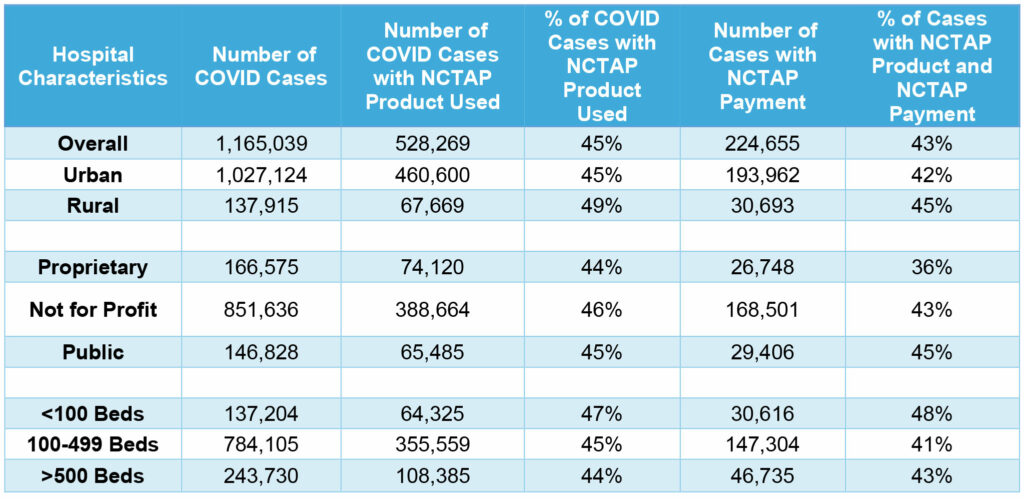
Table 1 shows the proportion of COVID-19 cases using NCTAP products and receiving additional NCTAP payments during the study period. When examining utilization trends of NCTAP products by hospital characteristics, ADVI found that rural hospitals utilize COVID-19 therapies at a higher proportion than urban hospitals (49% vs 45% of cases). Rural hospitals are also more likely to receive NCTAP payments than urban hospitals (45% vs 42% of cases that billed for NCTAP products). ADVI also found that small hospitals (<100 beds) were significantly more likely to use NCTAP products and receive NCTAP payments compared to the other two groups (combined). ADVI also found that Not for Profit hospitals were more likely to use NCTAP products compared to Proprietary and Public hospitals (combined); however, Public hospitals were more likely to receive NCTAP payments.3
Table 1: NCTAP Utilization by Hospital Characteristics
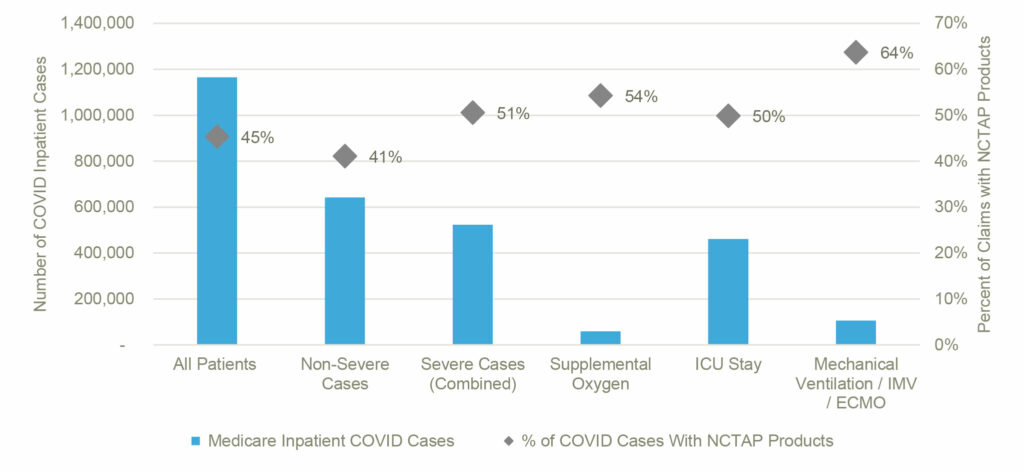
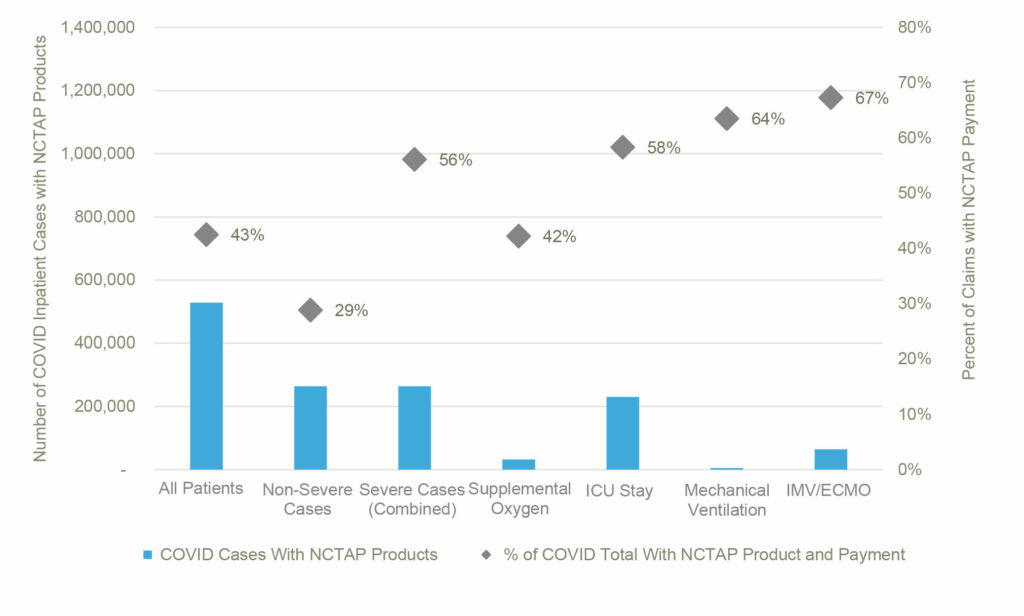
Severe cases, identified as cases with an ICU stay, mechanical ventilation/IMV/ECMO, and/or supplemental oxygen, made up 45% (n=522,767) of the total inpatient COVID-19 claims. NCTAP products were present on 51% of these cases, compared to 41% of the non-severe cases (as seen in Figure 3). The mechanical ventilation/IMV/ECMO cases had the highest proportion with NCTAP products (64%).
Figure 3: Frequency of NCTAP Product Usage Across Severe Claims (Combined) and By Type of Severity
Of the severe cases with NCTAP products, 56% received NCTAP payments, which is a higher rate than non-severe claims with NCTAP products and NCTAP payments (29%). Figure 4 (below) provides a summary of case volumes by indices of severity and demonstrates that as case severity increases, the likelihood of receiving a NCTAP payment increases.
Figure 4: Frequency of NCTAP Payment on Cases with NCTAP Products
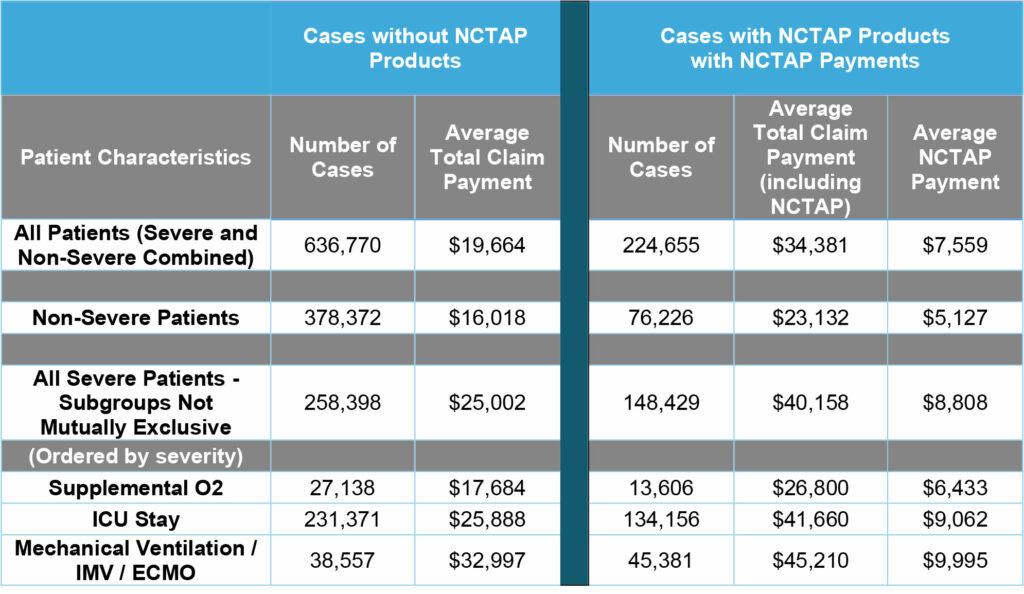
Average total claim payment and NCTAP payment with indices of severity are summarized in Table 2. This study found that regardless of patient severity, cases that utilize NCTAP products are paid, on average, more than cases that do not utilize NCTAP products. In addition, NCTAP payment increases with case severity. Claims with mechanical ventilation/IMV/ECMO saw the highest average NCTAP payment ($9,995).
Table 2: NCTAP Payments for COVID-19 Cases by Patient Severity
ADVI analyzed NCTAP utilization on a product-level basis to determine the most billed therapies. Table 3, below, shows the proportion of claims that utilize each product as well as the proportion of cases that received NCTAP payment when the product was used. Veklury® (remdesivir) is the most commonly used product in cases that qualify for and receive NCTAP payment.
Table 3: Proportion of Claims by NCTAP Products and Patient Severity
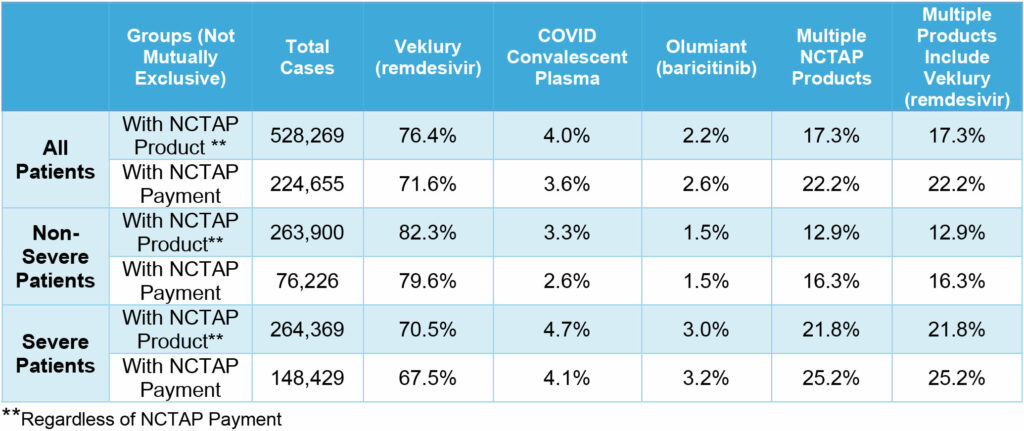
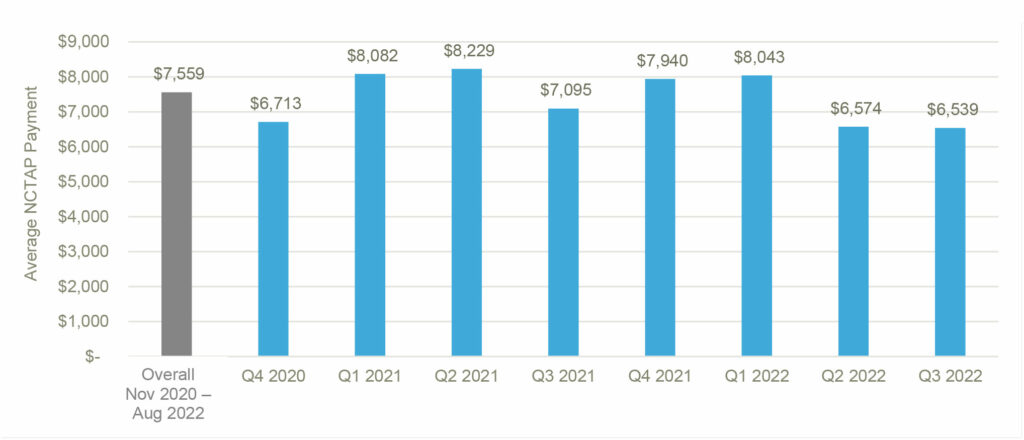
Figure 5 shows the average NCTAP payments over the study period by quarter. The average NCTAP payment fluctuated throughout the study period with an average NCTAP payment of $7,559.
Figure 5: Average NCTAP Payments Overall and By Quarter
Funding for this research was provided by Gilead Sciences. ADVI Health retained full editorial control.
Methodological Notes on NCTAP Products Used in Analysis
Several products are eligible for NCTAP payments that are only identifiable by NDC codes. Examples include Paxlovid (nirmatrelvir, ritonavir) and Lagevrio (molnupiravir). Inpatient claims typically do not contain NDC codes; however, the inpatient claims in this analysis were assessed for the applicable NDC codes, and none of the NCTAP drug products identifiable by NDC code were found. ADVI also assessed the prescription drug (Part D) claims for the beneficiaries and found 1,046,762 beneficiaries and 1,057,247 claims for Paxlovid (nirmatrelvir, ritonavir) from Dec 2021 to Aug 2022. Out of those claims, 2,056 claims fell within the date of an inpatient stay. These claims were not included in the analysis but may warrant further evaluation.
1 The NCTAP products were identified with the following ICD-10 procedure codes on the inpatient claims: remdesivir (XW033E5, XW043E5), COVID convalescent plasma (XW13325, XW14325), and baricitinib (XW0DXF5, 3E0G7GC, 3E0H7GC, XW0DXM6, XW0G7M6, XW0H7M6).
2 Variables for patient severity were identified by ICD-10 diagnosis codes (supportive oxygen [Z99.81], mechanical ventilation [Z99.0, Z99.11], IMV/ECMO [Z92.81]) as well as ICD-10 procedure codes (IMV/ECMO [14 applicable codes]).
3 All differences were statistically significant at p<0.0001.


Head of SAVEs